Comprehensive Guide to Apremilast: Uses, Dosage, Side Effects, and More
What is Apremilast?
Overview of Apremilast
Generic Name: Apremilast
Brand Name: Otezla, generics
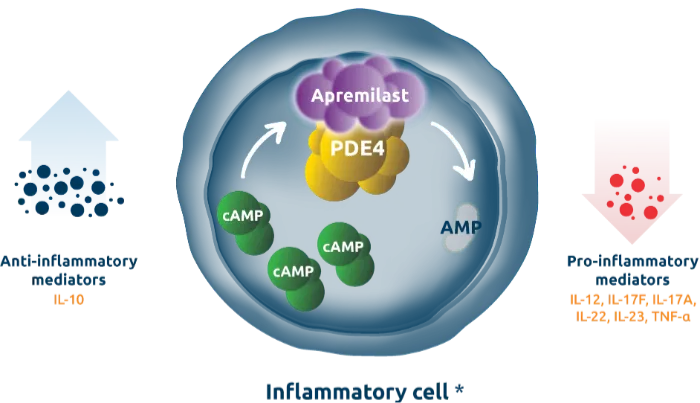
Drug Group: Phosphodiesterase-4 (PDE4) inhibitor
Commonly Used For
- Treat moderate to severe plaque psoriasis in adults.
- Manage active psoriatic arthritis in adults.
- Off-label uses include Behçet’s disease or ankylosing spondylitis under specialist guidance.
Key Characteristics
Form: Oral tablets (10 mg, 20 mg, 30 mg).
Mechanism: Inhibits PDE4, increasing cyclic AMP levels to reduce inflammation.
Approval: FDA-approved (2014 for Otezla) and EMA-approved for psoriasis and psoriatic arthritis.
Indications and Uses of Apremilast
Plaque Psoriasis: Treats moderate to severe cases in adults who are candidates for phototherapy or systemic therapy.
Psoriatic Arthritis: Reduces signs and symptoms in adults with active disease.
Off-Label Uses: Manages Behçet’s disease oral ulcers or other inflammatory conditions under specialist supervision.
Dosage of Apremilast
Dosage for Adults
Plaque Psoriasis and Psoriatic Arthritis:
- Titration Schedule: Start with 10 mg on Day 1, increase to 20 mg twice daily on Day 2, 30 mg twice daily on Day 3, and maintain 30 mg twice daily from Day 6 onward.
- Maintenance: 30 mg twice daily, with or without food.
Off-Label Use: Dosing varies; typically 30 mg twice daily, adjusted by a specialist.
Dosage for Children
Not Approved: Apremilast is not indicated for pediatric use due to lack of safety and efficacy data.
Dosage for Pregnant Women
Dosage Adjustments
Renal Impairment:
- CrCl ≤30 mL/min: Reduce to 30 mg once daily.
- Severe impairment (CrCl <15 mL/min): Avoid use.
Hepatic Impairment: No adjustment needed; monitor for side effects.
Elderly: No specific adjustment; monitor for tolerability.
Additional Considerations
- Take this active ingredient with or without food at consistent times.
- Follow the titration schedule to reduce gastrointestinal side effects.
How to Use Apremilast
Administration: Swallow tablets whole with water, with or without food.
Timing: Take twice daily, at the same times each day, following the titration schedule.
Monitoring: Watch for signs of depression or severe diarrhea.
Additional Tips:
- Avoid abrupt discontinuation; taper if stopping long-term use.
- Report mood changes or persistent gastrointestinal issues immediately.
Contraindications
This drug is contraindicated in:
- Patients with hypersensitivity to Apremilast or its components.
- Patients with severe renal impairment (CrCl <15 mL/min).
Warnings & Precautions
General Warnings
Depression and Suicide: Risk of mood changes; monitor for suicidal thoughts.
Gastrointestinal Effects: Common diarrhea or nausea; may require dose adjustment.
Weight Loss: Unintentional weight loss reported; monitor body weight.
Infections: Mild risk; avoid in active infections.
Drug Interactions: Limited; monitor with strong CYP enzyme inducers.
Use in Specific Populations
Pregnancy: Category C; limited data; use only if essential.
Breastfeeding: Excretion in breast milk unknown; avoid during treatment.
Elderly: Increased risk of side effects; monitor closely.
Children: Not approved; safety not established.
Renal Impairment: Adjust dose in moderate to severe cases; avoid in severe impairment.
Additional Precautions
- Inform your doctor about mental health history, kidney disease, or weight loss before starting this medication.
- Avoid live vaccines during treatment if possible.
Overdose and Management
Overdose Symptoms
- Severe diarrhea or nausea.
- Headache or dizziness.
- Mood changes or fatigue.
Immediate Actions
Contact Emergency Services: Seek medical help if overdose is suspected.
Supportive Care: Provide symptomatic treatment (e.g., hydration for diarrhea).
Monitor: Check renal function and mental status.
Additional Notes
- Overdose is rare with proper dosing; store securely.
- Report persistent symptoms promptly.
Side Effects of Apremilast
Common Side Effects
- Diarrhea (10–15%)
- Nausea (7–10%)
- Headache (5–8%)
- Upper respiratory infection (3–5%)
These effects may decrease over time.
Serious Side Effects
Psychiatric: Depression, suicidal thoughts, or severe mood swings.
Gastrointestinal: Severe abdominal pain or persistent vomiting.
Infections: Fever or signs of infection.
Weight Loss: Unexplained weight loss >5–10% of body weight.
Additional Notes
- Regular monitoring for mood and weight changes is recommended.
- Report any unusual symptoms immediately.
Drug Interactions with Apremilast
This active ingredient may interact with:
Strong CYP3A4 Inducers (e.g., Rifampin): Decrease Apremilast levels; avoid concurrent use.
Strong CYP3A4 Inhibitors: Limited effect; monitor if combined.
Immunosuppressants: Potential additive effects; use cautiously.
Antidepressants: Monitor for enhanced psychiatric effects.
Patient Education or Lifestyle
Medication Adherence: Take this PDE4 inhibitor twice daily as prescribed to manage symptoms.
Monitoring: Report mood changes, weight loss, or severe diarrhea immediately.
Lifestyle: Maintain a balanced diet and avoid excessive alcohol to support gastrointestinal health.
Diet: No specific restrictions; ensure adequate hydration.
Emergency Awareness: Know signs of depression or severe infection; seek care if present.
Follow-Up: Schedule regular visits to assess treatment response and side effects.
Pharmacokinetics of Apremilast
Absorption: Well-absorbed orally; peak plasma concentration at 2–3 hours.
Distribution: Volume of distribution ~87 L; 68–82% protein-bound.
Metabolism: Hepatic via CYP3A4 and CYP1A2 to inactive metabolites.
Excretion: Primarily urinary (58% as metabolites) and fecal.
Half-Life: 6–9 hours.
Pharmacodynamics of Apremilast
- Inhibiting PDE4, increasing cyclic AMP to reduce inflammation.
- Decreasing pro-inflammatory cytokines (e.g., TNF-α, IL-17) in psoriasis and arthritis.
- Providing symptom relief without immunosuppression.
- Effective for chronic management of inflammatory diseases.
Storage of Apremilast
Temperature: Store at 15–30°C (59–86°F); protect from moisture.
Protection: Keep in original container to shield from light.
Safety: Store out of reach of children.
Disposal: Dispose of unused tablets per local regulations or consult a pharmacist.
Frequently Asked Questions (FAQs)
Q: What does Apremilast treat?
A: This medication treats plaque psoriasis and psoriatic arthritis.
Q: Can this active ingredient cause depression?
A: Yes, mood changes are a risk; report suicidal thoughts immediately.
Q: Is Apremilast safe for children?
A: No, it is not approved for pediatric use.
Q: How is this drug taken?
A: Twice daily as an oral tablet, with a titration schedule.
Q: How long is Apremilast treatment?
A: Ongoing for chronic conditions; reassess every 3–6 months.
Regulatory Information
This medication is approved by:
U.S. Food and Drug Administration (FDA): Approved in 2014 (Otezla) for psoriasis and psoriatic arthritis.
European Medicines Agency (EMA): Approved for similar indications.
Other Agencies: Approved globally for equivalent uses; consult local guidelines.
References
- U.S. Food and Drug Administration (FDA). (2023). Otezla (Apremilast) Prescribing Information.
- Official FDA documentation detailing the drug’s approved uses, dosage, and safety.
- European Medicines Agency (EMA). (2023). Apremilast Summary of Product Characteristics.
- EMA’s comprehensive information on the medication’s indications and precautions in Europe.
- National Institutes of Health (NIH). (2023). Apremilast: MedlinePlus Drug Information.
- NIH resource providing detailed information on the drug’s uses, side effects, and precautions.
- World Health Organization (WHO). (2023). WHO Model List of Essential Medicines: Apremilast.
- WHO’s inclusion of Apremilast for inflammatory diseases.
- Journal of the American Academy of Dermatology. (2020). Apremilast in Psoriasis and Psoriatic Arthritis.
- Peer-reviewed article on Apremilast efficacy (note: access may require a subscription).
