Comprehensive Guide to Amikacin: Uses, Dosage, Side Effects, and More
What is Amikacin?
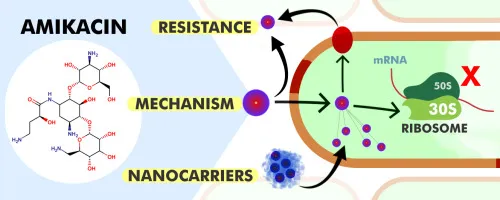
Overview of Amikacin
Generic Name: Amikacin
Brand Name: Amikin, generics
Drug Group: Aminoglycoside antibiotic
The medication is used to
- Treat serious Gram-negative bacterial infections (e.g., Escherichia coli, Pseudomonas aeruginosa, Klebsiella).
- Manage hospital-acquired infections, such as pneumonia or sepsis.
- Treat complicated urinary tract infections (UTIs) or intra-abdominal infections.
- Off-label uses include mycobacterial infections (e.g., Mycobacterium tuberculosis) under specialist guidance.
Key Characteristics
Form: Injectable solution (50 mg/mL, 250 mg/mL) for intravenous (IV) or intramuscular (IM) administration.
Mechanism: Binds to the 30S ribosomal subunit, inhibiting bacterial protein synthesis.
Approval: FDA-approved (1976 for Amikin) and EMA-approved for serious bacterial infections.
Indications and Uses of Amikacin
Amikacin is indicated for:
Gram-Negative Infections: Treats infections caused by susceptible bacteria, including Pseudomonas aeruginosa, Escherichia coli, Klebsiella, and Enterobacter.
Hospital-Acquired Infections: Manages ventilator-associated pneumonia, sepsis, or bacteremia in hospitalized patients.
Complicated Infections: Treats severe UTIs, intra-abdominal infections, or bone/joint infections.
Off-Label Uses: Adjunct therapy for multidrug-resistant tuberculosis or nontuberculous mycobacterial infections under specialist supervision.
Dosage of Amikacin
Dosage for Adults
Serious Infections:
- Standard: 15 mg/kg/day IV or IM, divided every 8–12 hours (maximum 1.5 g/day).
- Once-Daily Dosing: 15–20 mg/kg once daily (preferred for some infections to reduce toxicity).
Mycobacterial Infections (Off-Label): 10–15 mg/kg/day or 25 mg/kg three times weekly, adjusted per protocol.
Duration: Typically 7–14 days, depending on infection severity and response.
Dosage for Children
Neonates (≤4 weeks): 7.5–15 mg/kg every 12–24 hours, adjusted for gestational age and weight.
Children (≥1 month): 15–22.5 mg/kg/day, divided every 8–12 hours (maximum 1.5 g/day).
Dosage for Pregnant Women
Dosage Adjustments
Renal Impairment:
- Adjust dose or interval based on creatinine clearance (CrCl):
- CrCl 50–90 mL/min: 15 mg/kg every 12–24 hours.
- CrCl 10–50 mL/min: 15 mg/kg every 24–48 hours.
- CrCl <10 mL/min: Individualized dosing with drug level monitoring.
Dialysis: Administer post-dialysis; monitor serum levels.
Elderly: Lower doses or extended intervals due to reduced renal function; monitor closely.
Additional Considerations
- Monitor peak (30 minutes post-dose) and trough (pre-dose) serum levels to optimize efficacy and minimize toxicity (target peak: 20–35 mcg/mL; trough: <5 mcg/mL).
- Administer IV infusions over 30–60 minutes to reduce infusion-related reactions.
How to Use Amikacin
Administration:
- Intravenous (IV): Dilute in compatible fluid (e.g., normal saline); infuse over 30–60 minutes.
- Intramuscular (IM): Inject into large muscle (e.g., gluteus); rotate sites to avoid irritation.
Timing: Follow prescribed schedule (e.g., every 8–12 hours or once daily).
Monitoring: Regular blood tests for kidney function, hearing tests, and Amikacin levels are essential.
Additional Tips:
- Report signs of hearing loss (e.g., tinnitus), dizziness, or reduced urine output immediately.
- Ensure adequate hydration to protect kidney function.
Contraindications for Amikacin
Patients with hypersensitivity to Amikacin, other aminoglycosides, or components.
Patients with a history of severe ototoxicity or nephrotoxicity from aminoglycosides.
Warnings & Precautions for Amikacin
General Warnings
Ototoxicity: Risk of irreversible hearing loss or vestibular dysfunction; monitor with audiometric testing, especially with prolonged use.
Nephrotoxicity: Risk of kidney damage; monitor creatinine and urine output regularly.
Neuromuscular Blockade: Rare; may cause respiratory depression, especially with high doses or in patients with myasthenia gravis.
Antibiotic Resistance: Overuse may contribute to resistance; use only for confirmed susceptible infections.
Electrolyte Imbalance: Risk of hypomagnesemia or hypokalemia; monitor electrolytes.
Use in Specific Populations
Pregnancy: Category D; risk of fetal hearing loss; use only for life-threatening infections.
Breastfeeding: Excreted in breast milk in low amounts; use cautiously and consult a doctor.
Elderly: Higher risk of renal and hearing toxicity; adjust dose and monitor closely.
Children: Approved for neonates and children; monitor for toxicity.
Renal Impairment: High risk of toxicity; adjust dose and monitor drug levels.
Additional Precautions
- Inform your doctor about kidney disease, hearing problems, or neuromuscular disorders before starting the medication.
- Avoid concurrent use of other nephrotoxic or ototoxic drugs (e.g., vancomycin, loop diuretics).
Overdose and Management of Amikacin
Overdose Symptoms
- Hearing loss or tinnitus (ototoxicity).
- Reduced urine output or kidney failure (nephrotoxicity).
- Neuromuscular weakness or respiratory depression.
Immediate Actions
Contact Emergency Services: Stop infusion; seek immediate medical intervention.
Supportive Care: Monitor kidney function, hearing, and respiratory status; provide hydration or supportive care.
Hemodialysis: May remove Amikacin in severe cases; consult a specialist.
Additional Notes
- Overdose is rare with proper dosing and monitoring; ensure accurate weight-based calculations.
- Report persistent symptoms promptly.
Side Effects of Amikacin
Common Side Effects
- Nausea or vomiting (2–5%)
- Injection site pain (IM; 1–3%)
- Rash (1–2%)
- Fever (1–2%)
These effects are typically mild and resolve with treatment completion.
Serious Side Effects
Ototoxicity: Hearing loss, tinnitus, or vertigo.
Nephrotoxicity: Decreased urine output, swelling, or elevated creatinine.
Neuromuscular: Muscle weakness or respiratory difficulty.
Allergic Reactions: Rare; rash, hives, or anaphylaxis.
Additional Notes
- Regular monitoring of kidney function, hearing, and drug levels is critical.
- Report any unusual symptoms immediately.
Drug Interactions with Amikacin
The medication may interact with:
Nephrotoxic Drugs (e.g., Vancomycin, NSAIDs): Increase kidney damage risk; avoid or monitor closely.
Ototoxic Drugs (e.g., Loop Diuretics like Furosemide): Increase hearing loss risk; use cautiously.
Neuromuscular Blockers: Enhance neuromuscular blockade; avoid in patients with myasthenia gravis.
Cephalosporins (e.g., Cefepime): Potential for additive nephrotoxicity; monitor renal function.
Patient Education or Lifestyle
Medication Adherence: Amikacin is administered by professionals; follow hospital treatment protocols and attend follow-up visits.
Monitoring: Report symptoms like tinnitus, dizziness, or reduced urine output immediately. Regular blood and hearing tests are required.
Lifestyle: Stay hydrated to support kidney function; follow infection control measures (e.g., hand hygiene) to prevent further infections.
Diet: Maintain a balanced diet; avoid excessive potassium or magnesium supplements unless prescribed.
Emergency Awareness: Know signs of ototoxicity (hearing changes) or nephrotoxicity (swelling, low urine output); seek immediate care if present.
Follow-Up: Schedule post-treatment tests to assess kidney and hearing function.
Pharmacokinetics of Amikacin
Absorption: Negligible oral absorption; administered IV or IM for systemic effect.
Distribution: Volume of distribution ~0.2–0.3 L/kg; poor penetration into cerebrospinal fluid.
Metabolism: Not metabolized; excreted unchanged.
Excretion: Primarily renal (>90% unchanged via glomerular filtration).
Half-Life: 2–3 hours (prolonged in renal impairment).
Pharmacodynamics of Amikacin
The antibiotic exerts its effects by:
Binding to the bacterial 30S ribosomal subunit, inhibiting protein synthesis.
Exhibiting bactericidal activity against Gram-negative bacteria and some Gram-positive organisms.
Demonstrating concentration-dependent killing, optimized with high peak levels.
Effective against multidrug-resistant strains when susceptibility is confirmed.
Storage of Amikacin
Temperature: Store at room temperature (15–25°C or 59–77°F); avoid freezing.
Protection: Keep vials in original packaging to protect from light.
Safety: Store out of reach of children; restricted to hospital use.
Disposal: Dispose of unused solutions or vials per hospital protocols and local regulations.
Frequently Asked Questions (FAQs)
Q: What does Amikacin treat?
A: The drug treats serious Gram-negative bacterial infections, such as pneumonia or sepsis.
Q: Can Amikacin cause hearing loss?
A: Yes, ototoxicity is a risk; report tinnitus or hearing changes immediately.
Q: Is Amikacin safe for children?
A: Approved for neonates and children with adjusted dosing; monitor for toxicity.
Q: How is Amikacin administered?
A: Via IV infusion or IM injection in a hospital by trained professionals.
Q: How long is Amikacin treatment?
A: Typically 7–14 days, depending on infection severity and response.
Regulatory Information
The medication is approved by:
U.S. Food and Drug Administration (FDA): Approved in 1976 (Amikin) for serious bacterial infections.
European Medicines Agency (EMA): Approved for similar indications.
Other Agencies: Approved globally for equivalent uses; consult local guidelines.
References
- U.S. Food and Drug Administration (FDA). (2023). Amikin (Amikacin) Prescribing Information.
- Official FDA documentation detailing the drug’s approved uses, dosage, and safety.
- European Medicines Agency (EMA). (2023). Amikacin Summary of Product Characteristics.
- EMA’s comprehensive information on the medication’s indications and precautions in Europe.
- National Institutes of Health (NIH). (2023). Amikacin: MedlinePlus Drug Information.
- NIH resource providing detailed information on the drug’s uses, side effects, and precautions.
- World Health Organization (WHO). (2023). WHO Model List of Essential Medicines: Amikacin.
- WHO’s inclusion of Amikacin for serious bacterial infections.
- Clinical Infectious Diseases. (2020). Aminoglycosides in Multidrug-Resistant Infections.
- Peer-reviewed article on Amikacin efficacy (note: access may require a subscription).
